GKL
Forum Supporter
Here are pictures of the last 3 fluorescent specimens that KT brought back to the Castle from the Jackson, MS, Gem & Mineral Show last weekend.
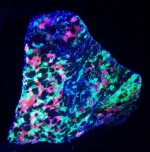
The first specimen is a handsized piece of bladed white calcite, location given as Mexico. Since there are several dozen sites in Mexico that contain collectable calcite, KT attempted to find one that contains bladed fluorescent white calcite, but was unsuccessful. Every Mexican site that had anything looking similar all fluoresce red! So I will leave the site as simply Mexico. The first picture is in natural light and the second in LWUV 365nm.
The second pair of images are of silicified coral from north central Florida, another kind of vague location. But KT looked through a box with a couple of dozen specimens of this material and this one was absolutely the best piece. It is a bit smaller than the calcite, and the translucent brownish tan chalcedony is undamaged. It sits attractively too. First image is in natural light and the second image is in LWUV 365nm. It fluoresces a brilliant white.
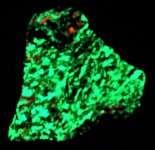
The final specimen is of hyaline, a water clear variety of opal, as a thin coating on pegmatitic feldspar from Spruce Pine mine, Mitchell Co., NC. The first picture is in natural light and the second in UVLW 365nm. The brilliant green color is due to traces of Uranium captured in the opal. It is a flat specimen measuring ~3.5" long. KT had a self collected specimen of this material but when the Royal Collection sold, it went with it. At that time KT was not interested particularly in fluorescent minerals, so His Majesty is happy to have another nice example in the Royal Collection!
Hope you enjoy the pictures!
Interesting contrast of textures between the first two KT !!!!
Definitely not your average looking rocks !
The one with the eerie green glow due to "traces of Uranium" - are you sure you don't need a Geiger counter ?
 (just joking
(just joking  )
)Enjoyed the pics KT, thanks for posting those !







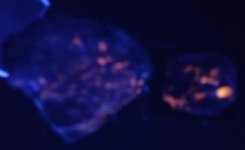

 thanks to you !!!!
thanks to you !!!!