fdutra
Full Member
Here you go Kapidr,
I've tried to keep this simple and while it may well horrify those of the scientific community, it will work just fine for us mere earthy types that just want to know what type and/or karat metal that recently rescued jewelry is made of. If you own a gram scale that can handle 50 grams and will read at least one decimal place (.1 g), you have everything you need to get going. While an acid test will do a lot of what we need, Specific Gravity (SG) can be used to identify just about any elemental metal and reveal gold filled or plated jewelry, as well as other imposters. If the word "Math" doesn't make you swoon, you can also determine weights and types of mounted gemstones (1+ carat) and two part alloys where one metal is known.
Ingredients:

One digital scale
One small plastic cup (cut down plastic spice jar)
Water (tap is fine)
Dental floss, or just plain old thread
Ring or other jewelry item
Pen and paper or calculator
Step 1

Turn on scale and weigh ring, recording wt on paper or calculator.
Step 2
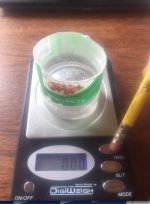
Fill cup with enough water to cover ring vertically, place on scale and tare.
(record weight if your scale doesn't have the tare option)
Step 3

Dangle ring from string lowering into cup until the ring is completely submerged, being careful not to hit the sides or bottom of the cup or splash water around, record weight of ring in water. This is the hardest part,,, your almost done!
Step 4
Divide the weight of the ring (step 1) by the weight of the ring in water (Step 3), the answer is your experimentally derived Specific Gravity for that ring. Thats all she wrote, your now a fledgling scientist with your very own data set and unique specific gravity value. All thats left is to compare your numbers with ones on the internet for your suspected (hopeful?) metal. You should be able to at least get within shouting distance (+ or minus 1) with a one decimal place scale and some care in step 3. A two decimal place scale usually gets me a little closer to the given values (+ or minus .5), spend a fortune on 5 decimal place scales and weighing devices and your still only going to be out to 2 decimal point accuracy. If you test a ring and it comes in at 10 its roughly 90% silver, could be sterling. If it's 10.3 it's definitely 925 sterling silver, What about 10.36 ? Still 925 sterling silver. The silvery ring (marked 14 k) in step 2 and 3 came in at 12.56 (2 decimal place scale), the listed value for 14 k white gold is 12.61,,, nailed it out to 2 places.
http://www.hauserandmiller.com/reference/melting.html

Thanks for looking and feel free to ask questions. There's a lot more you can do with SG and ways to be a little more accurate, but this should get you going. For fun I did up a dozen different rings in about a half hour. If anyones interested I can post them up to show how amazingly accurate and useful this method is in real life, but to supper I must go,,, took me about a half day just to get the pictures posted in the right orientation! Still blowing a gale and maybe I won't be able to go scalloping again tomorrow, but I just might be able to get out to some newly eroded beaches on the lee side and shovel my thesis!
Happy Hunting All!
I've tried to keep this simple and while it may well horrify those of the scientific community, it will work just fine for us mere earthy types that just want to know what type and/or karat metal that recently rescued jewelry is made of. If you own a gram scale that can handle 50 grams and will read at least one decimal place (.1 g), you have everything you need to get going. While an acid test will do a lot of what we need, Specific Gravity (SG) can be used to identify just about any elemental metal and reveal gold filled or plated jewelry, as well as other imposters. If the word "Math" doesn't make you swoon, you can also determine weights and types of mounted gemstones (1+ carat) and two part alloys where one metal is known.
Ingredients:

One digital scale
One small plastic cup (cut down plastic spice jar)
Water (tap is fine)
Dental floss, or just plain old thread
Ring or other jewelry item
Pen and paper or calculator
Step 1

Turn on scale and weigh ring, recording wt on paper or calculator.
Step 2

Fill cup with enough water to cover ring vertically, place on scale and tare.
(record weight if your scale doesn't have the tare option)
Step 3

Dangle ring from string lowering into cup until the ring is completely submerged, being careful not to hit the sides or bottom of the cup or splash water around, record weight of ring in water. This is the hardest part,,, your almost done!
Step 4
Divide the weight of the ring (step 1) by the weight of the ring in water (Step 3), the answer is your experimentally derived Specific Gravity for that ring. Thats all she wrote, your now a fledgling scientist with your very own data set and unique specific gravity value. All thats left is to compare your numbers with ones on the internet for your suspected (hopeful?) metal. You should be able to at least get within shouting distance (+ or minus 1) with a one decimal place scale and some care in step 3. A two decimal place scale usually gets me a little closer to the given values (+ or minus .5), spend a fortune on 5 decimal place scales and weighing devices and your still only going to be out to 2 decimal point accuracy. If you test a ring and it comes in at 10 its roughly 90% silver, could be sterling. If it's 10.3 it's definitely 925 sterling silver, What about 10.36 ? Still 925 sterling silver. The silvery ring (marked 14 k) in step 2 and 3 came in at 12.56 (2 decimal place scale), the listed value for 14 k white gold is 12.61,,, nailed it out to 2 places.
http://www.hauserandmiller.com/reference/melting.html

Thanks for looking and feel free to ask questions. There's a lot more you can do with SG and ways to be a little more accurate, but this should get you going. For fun I did up a dozen different rings in about a half hour. If anyones interested I can post them up to show how amazingly accurate and useful this method is in real life, but to supper I must go,,, took me about a half day just to get the pictures posted in the right orientation! Still blowing a gale and maybe I won't be able to go scalloping again tomorrow, but I just might be able to get out to some newly eroded beaches on the lee side and shovel my thesis!
Happy Hunting All!
Last edited:


 I'll definitely be referring to this in the future. Thanks for sharing
I'll definitely be referring to this in the future. Thanks for sharing 
