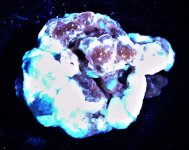
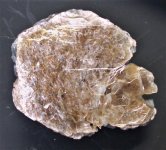
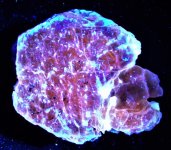
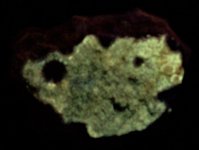
GKL
Forum Supporter
Believe it or not, there has been and still is a lot of research into the invention of a new type of SW UV emitter, but up til now no one has been able to get past a tube situation…until a few years back LW UV was stuck in the same situation, until the invention of the CREE led and new types of LW UV filters, then several portable very powerful 365 nm flashlights appeared on the scene. But for now we just have to have patience until some innovative inventor finds the right combination! So, tho we may dream of such a lamp, it is not yet available!
Hope you enjoyed the animation

Actually just doing some online research today and it seems they do have a SW LED flashlight available, just not in the USA, seems like it's unavailability in the USA is due to a patent dispute.
Here is a discussion thread where I read some info about it -
https://www.reddit.com/r/flashlight/comments/q0hdo0/worlds_first_shortwave_255nm_flashlight_that_you/
Hopefully we can find a way to buy them, and they are not super expensive